EGFR PDX Study
EGFR Mutant Tumors for PDX Study

Pasi A. Jänne, MD, PhD
Dana-Farber Cancer Institute
EGFR mutations are detected in approximately 15% of all patients diagnosed with lung cancer. There are several types of EGFR mutations including both the common L858R and exon 19 deletions (accounting for 85%) or the rare exon 20 insertion (accounting for 5-8%) EGFR mutations.
Different types of therapies are being used for these two groups of EGFR mutations. Osimertinib is an EGFR inhibitor approved for patients newly diagnosed with EGFR exon 19 or L858R mutations and for patients who have been treated with a prior EGFR inhibitor but have developed EGFR T790M as a resistance mechanism. In contrast, there are no approved EGFR inhibitors for patients with EGFR or HER2 exon 20 insertion mutations although several therapies are under evaluation in clinical trials.
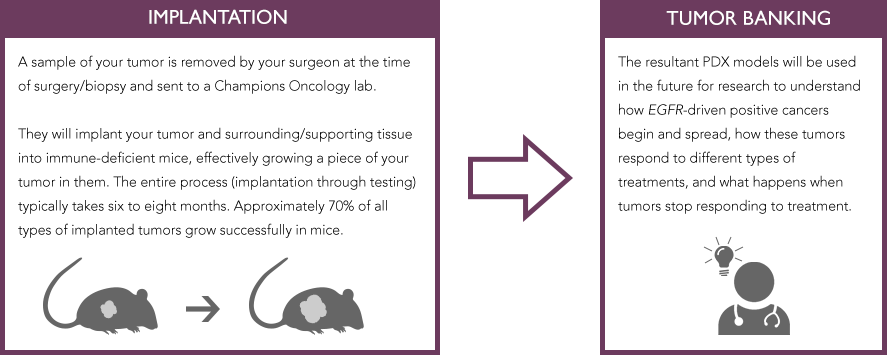
The Addario Lung Cancer Medical Institute (ALCMI) would like to focus on studying the cancers of patients previously treated with osimertinib or those with EGFR or HER2 exon 20 insertion mutations. The goal is to better understand how these tumors respond to drugs, and what happens when tumors stop responding to drugs. By studying these cancers we hope to accelerate the development of new therapeutic approaches for patients with EGFR mutant lung cancer.
Click on topic below for more information.